![]() You don't need to be an 'investor' to invest in Singletrack: 6 days left: 95% of target - Find out more
You don't need to be an 'investor' to invest in Singletrack: 6 days left: 95% of target - Find out more
My 6 yr old asked me a question I couldn't answer. How do they squeeze so much air into a cartridge and seal it before it all escapes again?
Any answers without using Google?
Well, for a start they fill it with CO2, not air.
liquid CO2 ?
Cool it until it's liquid?
Well, for a start they fill it with CO2, not air.
Give her a break, she's only 6!
Why bother liquifying the CO2? Just use and evacuated canister and connect a gaseous supply at pressure. You won't get liquid CO2 at those pressures and ambient temperatures.
It's chilled until it's a liquid then pumped into the cartridge then sealed.
Put the lid on really fast?
CO2 sublimates, ie goes from solid to gas...no liquid phase.
Cool it until it’s liquid?
If you cool CO2, it becomes a solid.
If you want it liquid, it needs to be under pressure.
Give her a break, she’s only 6!
OK, in plain terms. If you blow into it you will fill the cartridge with CO but it you blow really hard it will be full of CO2. At six years old you just won’t be able to blow hard enough
CO2 sublimates, ie goes from solid to gas…no liquid phase.
Uvavu!
At pressures higher than atmospheric CO2 resorts to a liquid form, IIRC around 14 bar. We keep copious stocks of liquified CO2 at work at roughly 21 bar, it's also how it is bulk transported in tankers.
The bulbs are probably filled by liquid weight and then quickly sealed (hence why they are referred to by their gram weight). The liquid will then gas off when the cylinder is pierced.
Bruneep's video was nice but it doesn't actually explain the question the OP's daughter had. I've got a little steel bottle full of CO2 (at high pressure, possibly as a liquid), how to I get the metal lid fitted and sealed on before the gas all escapes (especially since I guess the lid is welded on?). It went in a machine in the video which filled and sealed it but we didn't see the magic.
There is a ball in a tube, when compressed gas goes in, the ball is pushed in towards the bottle allowing gas to flow past. Once the gas is disconnected the pressure inside the bottle pushes the ball back to the end of the tube sealing the gas in by its own pressure.
That's my understanding. It may not be quite right but it explains a self sealing pressurised container
Which is fine but gas bulbs aren't self sealing. There is no ball inside. Life would be so much easier if they were.
I wonder if it works like our charge machine; one turret fills the bulb then another turret spins over, at pressure, and seals the tube.
Ha! A six year old asks a question that stumps STW!
It had to happen one day.
Politics. Sorted.
Covid. Sorted.
Brexit. Sorted.
Religion. (an uneasy truce)
Co2 into a little bottle? We are buggered.
I don't know how they actually do it, but I imagine you could do it by chilling the cartridge in liquid nitrogen, dropping in a chunk of frozen (solid) CO2, then using a machine to press a pre-soldered plug into the end and electrically heat that just enough to solder it and seal it. The CO2 would stay solid until the capping was finished.
Don't know but I do know that going from solid phase to gaseous phase without liquid phase is called sublimation.
To go from gaseous to solid is also called sublimation.
From Wikipedia:
The reverse process of sublimation is deposition or desublimation, in which a substance passes directly from a gas to a solid phase
When is her birthday? Get her a machine and let her take it apart? Quality bonding time?
Brocock used to make 12g-sized reuseable "CO2" cartridges for CO2 airguns. Filled with compressed air they were, and there was a valve. Not as good as actual CO2.
My 6 yr old asked me a question I couldn’t answer. How do they squeeze so much air into a cartridge and seal it before it all escapes again?
I work in the gas industry so I was interested in this question.
Firstly, as some others have said they don't put air in the cartridges, they use liquid CO2. When CO2 is pressurised enough it turns into liquid - typically this happens at about 50 times atmospheric pressure (about 750psi), the exact pressure varies with how warm the environment is that day. Becasue the CO2 is a liquid you get a lot more in the cartridge than using a gas. Gases like air do not turn into liquid at high pressures, which is why they are not suitable for this.
To fill the cartridge, the top of the open cartridge is pushed into a machine so it can work at pressure. Pressurised liquid CO2 is pumped from a cylinder or small tank into the cartridge. I think the same machine then inserts a thin metal plug into the top of the cartridge to seal it, before the cartridge is released.
The liquid CO2 does not totally fill the cartridge, there needs to be space for it to expand on hot days otherwise it could burst the cartridge if it got too hot. There are designs for refillable CO2 cartridges for use with air guns, but they a more expensive because they include a special valve device for refilling them and need equipment to be filled - too expensive for cyclists using a handful a year.
I always assumed it was super critical, but apparently not.
Makes sense, boiling liquids get cold, expanding super critical fluids get really cold which probably wouldn't do the tyre any good!
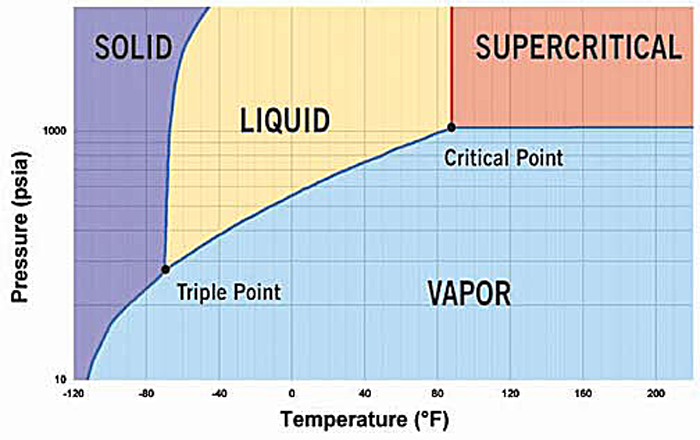
The phase diagram for CO2 suggests that it's on the fine line between liquid and gas at the typical cartridge pressure, but it is a fine line.
Just lobbed a CO2 cartridge in the freezer to see if the liquid CO2 sloshes when it gets a bit colder. Joys of lockdown science experiments.
Flaperon I just had a look at this (TINAS I just created a stream in HYSYS, the joys of home working) and it is unlikely that you'll get liquid CO2 from a domestic freezer. You'd need temps of around -40 to -30 C depending on the pressure, I assumed around 120 - 170 psig to get that temperature range. You might get some other liquids forming (from impurities) but I'd think liquid CO2 is unlikely.
It won't, at least probably not so you would notice. 8g isn't a lot and those cartridges are heavy, you may feel it when shaking but no more than at ambient temperature.
@J-R I take it the cap is stamped on and it's basically a bursting disc?
I've heard of the refillable 12g but wasn't sure it was Brocock that made them, I thought that was some French guy or was that the 8g recycler? Brocock definitely made the reusable all in one case that got banned over here because someone shat it that they could be converted to proper ammunition, you can get them in Spain still. 12g ASA refillable is only viable because of the ridiculous cost of the cartridges and it would be easier to just go HPA and retune anyway.
I always assumed it was super critical, but apparently not.
On a hot day, over about 88degF or 31C, it will become supercritical fluid instead of a liquid and gas mixture - as your phase diagram shows. The pressure by then will be over 1000psi.
phase diagram for CO2 suggests that it’s on the fine line between liquid and gas
- Yes it is exactly on that line because the canister contains liquid and a little gas. As the external temperature goes up or down, the pressure in the canister goes up and down along that line.
Just lobbed a CO2 cartridge in the freezer to see if the liquid CO2 sloshes when it gets a bit colder.
It's liquid CO2 in there, at all temperatures up to about 90F.
The question was How to get air in a CO2 cartridge
The answer to that is very simple, you use it.
If you blow into it you will fill the cartridge with CO but it you blow really hard it will be full of CO2.
what percentage of the air you breath out is co2? 😉
This thread defines exactly why I love this site! Excellent stuff. 😎
The question was How to get air in a CO2 cartridge. The answer to that is very simple, you use it.
Well done Dave - clearly the correct answer.
. At six years old you just won’t be able to blow hard enough
I think this was the best answer!
TINAS I just created a stream in HYSYS, the joys of home working
If you were really bored you would have tried it in ProII, before giving up after a couple of hours and realizing that ProII licences are only bought by middle management types trying to save money and wondering why on earth graduates can't run a simulation designed in FORTRAN and why they insist on using expensive HYSYS licences with their nice* GUI and easy to understand DOF indicators.
*it still looks like it's come out of windows 3.1 but at least it's not text based.
If you blow into it you will fill the cartridge with CO
Say what now?
I have fond memories of ProII, I’m a lot older than you. It may be text based but that makes the QA much easier
Couldn't you just put the canister in a chamber of some sort, fill the chamber with co2 at the right pressure, have some machine put a lid on the canister then depressurise the chamber? No need to faff around liquidizing it
Couldn’t you just put the canister in a chamber of some sort, fill the chamber with co2 at the right pressure, have some machine put a lid on the canister then depressurise the chamber? No need to faff around liquidizing it
From the description above, I think the "right pressure" would liquidize it.